Background: Single-agent gilteritinib and quizartinib (quiz) met their primary endpoints of improved overall survival (OS) in relapsed/refractory (R/R) FLT3-mut AML in phase III studies, but CRc durations were short (4-11 months). Quiz demonstrated potent synergy with venetoclax (VEN) (a BCL-2 inhibitor) in AML cell lines and PDX models (Mali et al. Haematologica 2020).
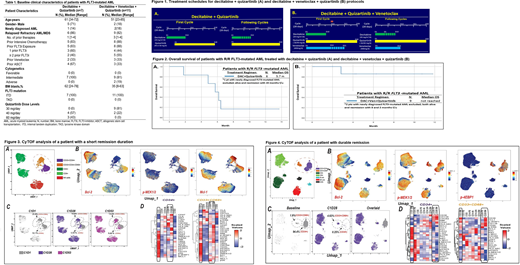
Methods: Newly diagnosed or R/R AML with ECOG ≤2, and adequate organ function were eligible. The protocol initially evaluated safety of the doublet of decitabine (DAC) plus quiz regardless of FLT3 status [Fig 1A]. After the first 10 pts were treated on the doublet, the protocol was amended to add VEN (triplet) in a safety dose escalation lead in [Fig 1B]. CRc criteria were as published in the phase III ADMIRAL and QUANTUM-R studies.
Results: 21 pts including DAC10 + quiz (phase 1: N=10) and DAC10 + VEN + quiz (phase IB/II: n=11) were evaluable at the time of this report (Table 1).
DAC10 + Quiz
Of 10 pts treated with DAC10 + quiz (Table 1), CRc rate was 40% (1 CRp and 3 CRi). No FLT3 R/R WT (n=3) pts responded. Four of 7 FLT3-ITD mut pts (1 frontline and 5/6 R/R, who had all received ≥1 FLT3 TKI) achieved CRc (57%), with 3/6 FLT3-PCR negative at response. 1 pt experienced QTcF prolongation > 500 msec on quiz 40mg/day (resolved after holding quiz with no clinical cardiac events). Grade 3/4 toxicities in >/= 2 pts, irrespective of attribution, included infection (12), neutropenic fever (3), mucositis (3), diarrhea (2), and prolonged QTcF >450 (2). With a median (med) follow-up (f/u) of 12 months (mos), the med OS was 5.7 months in the 6 R/R FLT3-mut pts (Fig 2A). Three of 4 CRc pts proceeded to allogeneic stem cell transplantation (ASCT) and 2 were alive in remission at last f/u.
DAC10 + VEN + Quiz
Of 11 enrolled pts (Table 2), 10 treated prior to July 1 2020 were evaluable. CRc achieved in 9/10 (90%) (1 CR, 3 CRp, 5 CRi) with 5/9 responders FLT3-PCR negative. Excluding the 1 frontline evaluable pt, 8 of 9 R/R FLT3-ITD (88% with ≥1 prior FLT3 TKIs) pts achieved CRc. No pts developed a DLT with 30 mg/day quiz, however with the 40mg/day quiz 2 pts developed hematologic DLT (grade ≥3 neutropenia with a <5% cellular bone marrow lasting ≥42 days. Hence, quiz 30 mg/day dose was determined as RP2D for triplet. Grade 3/4 toxicities in >/=2 pts, irrespective of attribution, included infection (7) and neutropenic fever (4). No QTcF prolongations >500msec noted. 60-day mortality was 0. With a med f/u of 6 mos, med OS was not reached and 6-month OS was 86% (one death) (Fig 2B). Of 9 CRc pts, 5 remain on protocol in CRc, 2 underwent ASCT and are alive post-ASCT, and 2 have relapsed.
CyTOF (single-cell mass cytometry) analysis was performed in longitudinal samples collected on the triplet therapy. In a pt (baseline FLT3-ITD, NRAS, IDH1/2, SRSF2, DNMT3A, RUNX1, CSF3R) with a 3 week CRc duration, we identified two distinct blast populations at C1D1; monocytic (CD33+/CD34-/CD68+) and myeloid (CD33+/CD34+) (Fig 3A). The monocytic blasts had low BCL-2 and high MCL-1 at baseline, with modest reduction at EOC1 (from 25.8% to 10.8%) (Fig 3B-C). However, the myeloid blasts with high BCL-2 and lower MCL-1 showed significant reduction at EOC1 (from 52% to 5.5%) (Fig 3B-C). Heat-map demonstrated major downregulation in signaling pathways (MCL-1, STAT, BCL-XL, and MAPK) in the myeloid blasts at EOC1 of triplet therapy (Fig 3D). However, these signaling pathways remained active in the monocytic blasts at EOC 1 (Fig 3D). Pt relapsed prior to starting C2, driven by monocytic blasts (Fig 3C).On the other hand, in a pt (baseline FLT3-ITD only) who remains in CR (DOR 11 mos), both myeloid and monocytic populations had high BCL-2 at baseline (Fig 4A-B). Despite almost complete elimination of myeloid blasts (Fig 4C), monocytic blasts were detectable at a low level at C1D28 (Fig 4C). Importantly, converse to the prior pt, the active signaling pathways at diagnosis (MCL-1, STAT, MAPK, c-MYC) were significantly downregulated in both populations at EOC1 (Fig 4D). This patient underwent ASCT on D35 of therapy, and remains in CR.
Conclusion: DAC10 + venetoclax + quiz is highly active in R/R FLT3-ITD mut AML pts, with CRc rates of 90% and projected 6-month OS of 86%. CYTOF profiling may predict for response based on pre- and on-therapy apoptotic and signaling pathway profiles enabling potential optimal selection of future combinatorial or sequential approaches. Accrual to the triplet continues and updated clinical and correlative data will be presented.
Yilmaz:Pfizer: Research Funding; Pint Pharma: Honoraria; Daicho Sankyo: Research Funding. Kantarjian:Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Research Funding; Takeda: Honoraria; BMS: Research Funding; Amgen: Honoraria, Research Funding; Jazz Pharma: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Research Funding; Pfizer: Honoraria, Research Funding; Cyclacel: Research Funding; Astex: Research Funding; Immunogen: Research Funding; Ariad: Research Funding. Kadia:Cellenkos: Research Funding; Cyclacel: Research Funding; Amgen: Research Funding; JAZZ: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria; Pulmotec: Research Funding; Abbvie: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Incyte: Research Funding; Astellas: Research Funding; Astra Zeneca: Research Funding; Celgene: Research Funding. Konopleva:Eli Lilly: Research Funding; Amgen: Consultancy; Cellectis: Research Funding; Agios: Research Funding; AbbVie: Consultancy, Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Forty-Seven: Consultancy, Research Funding; Sanofi: Research Funding; Rafael Pharmaceutical: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Research Funding; Ascentage: Research Funding; Stemline Therapeutics: Consultancy, Research Funding; Calithera: Research Funding; Ablynx: Research Funding; Kisoji: Consultancy. Borthakur:PTC Therapeutics: Research Funding; Incyte: Research Funding; Novartis: Research Funding; Abbvie: Research Funding; Jannsen: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; BioLine Rx: Research Funding; BMS: Research Funding; AstraZeneca: Research Funding; Polaris: Research Funding; Xbiotech USA: Research Funding; Oncoceutics: Research Funding; Curio Science LLC: Consultancy; FTC Therapeutics: Consultancy; Argenx: Consultancy; PTC Therapeutics: Consultancy; BioLine Rx: Consultancy; BioTherix: Consultancy; Nkarta Therapeutics: Consultancy; Treadwell Therapeutics: Consultancy. DiNardo:Takeda: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; ImmuneOnc: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Notable Labs: Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Calithera: Research Funding; Syros: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; MedImmune: Honoraria. Pemmaraju:Roche Diagnostics: Honoraria; Samus Therapeutics: Research Funding; SagerStrong Foundation: Other: Grant Support; MustangBio: Honoraria; Celgene: Honoraria; Pacylex Pharmaceuticals: Consultancy; Novartis: Honoraria, Research Funding; Incyte Corporation: Honoraria; AbbVie: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Blueprint Medicines: Honoraria; DAVA Oncology: Honoraria; Affymetrix: Other: Grant Support, Research Funding; Plexxikon: Research Funding; Cellectis: Research Funding; LFB Biotechnologies: Honoraria; Stemline Therapeutics: Honoraria, Research Funding. Short:Amgen: Honoraria; Takeda Oncology: Consultancy, Honoraria, Research Funding; Astellas: Research Funding; AstraZeneca: Consultancy. Alvarado:Daiichi-Sankyo: Research Funding; Tolero Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; Jazz Pharmaceuticals: Research Funding; FibroGen: Research Funding; Astex Pharmaceuticals: Research Funding; BerGenBio ASA: Research Funding; MEI Pharma: Research Funding. Jabbour:Takeda: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding. Garcia-Manero:Merck: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy; Amphivena Therapeutics: Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; H3 Biomedicine: Research Funding; Onconova: Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding. Ravandi:AstraZeneca: Consultancy, Honoraria; Orsenix: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Xencor: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; Astellas: Consultancy, Honoraria, Research Funding. Andreeff:Centre for Drug Research & Development; Cancer UK; NCI-CTEP; German Research Council; Leukemia Lymphoma Foundation (LLS); NCI-RDCRN (Rare Disease Clin Network); CLL Founcdation; BioLineRx; SentiBio; Aptose Biosciences, Inc: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Daiichi-Sankyo; Jazz Pharmaceuticals; Celgene; Amgen; AstraZeneca; 6 Dimensions Capital: Consultancy; Daiichi-Sankyo; Breast Cancer Research Foundation; CPRIT; NIH/NCI; Amgen; AstraZeneca: Research Funding. Daver:Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.